Members
Our members make us what we are
In Memoriam - Jerry Pelletier
It is with profound sorrow that we announce the passing of Dr. Jerry Pelletier, esteemed James McGill Professor in the Departments of Biochemistry and Oncology at McGill University and a member of the MRCCT and The Rosalind and Morris Goodman Cancer Research Institute. He was renowned for his groundbreaking contributions to the field of RNA biology and was a co-founder of the prestigious McGill Centre for RNA Sciences.
In 2017, Prof. Pelletier was honoured as a fellow of the Royal Society of Canada and in 2019 he was the recipient of the Robert L. Noble Prize from the Canadian Cancer Society for his groundbreaking work in elucidating the initiation and control of protein synthesis.
Prof. Pelletier’s journey in the field of RNA biology and therapeutics is a testament to his unwavering commitment to scientific research and unyielding curiosity up to his roles as a distinguished professor, co-founder of a biotech company, recipient of numerous awards, and co-founder of McGill’s Centre for RNA Sciences, his life reflects a tapestry of diverse experiences and groundbreaking discoveries.
Our condolences go out to Prof. Pelletier’s family, friends, colleagues and all those whose lives he touched. He will be greatly missed but his legacy will endure (Text by: Daniel Caron)
Multidisciplinary collaborations are fertile ground for breakthrough research
The diversity of our members’ expertise and interests makes the MRCCT an inspiring and opportune environment for innovative research.
To build on the latest advancements in genomics, the MRCCT brings together expertise in Medicine, Human Genetics, Microbiology and Immunology, Biochemistry and Physiology, Immunology, Microbial Physiology, Microbiology, Bioinformation, Chemistry, Computational Biology, Statistical Genetics, Population Genetics, Mouse Genetics, Immunology, Applied and Translational Genomics, Vaccinology, and Functional Genomics
Directors

Jorg Fritz
DirectorDr. Jörg Hermann Fritz, PhD, is a CIHR New Investigator, and an Associate Professor (Departments of Microbiology and Immunology and Physiology). He is an immunologist who trained with Dr. D. Philpott (Institut Pasteur, Paris), and Dr. J. Gommerman (U. Toronto), and who joined the MRCCT in 2010. Research in his laboratory focuses on understanding how tissue homeostasis at mucosal surfaces is regulated in health and disease. A particular focus is given to studies of mucosal pathogens of the respiratory and gastrointestinal system where he is trying to understand how the expression pattern and activity of innate resistance effectors adapts to changes in the tissue milieu due to the availability of nutritional metabolites, the composition of the mutualistic microflora, or infection with pathogens. In this context he is studying the priming and function of innate lymphoid cells and B lineage cells for their role in mucosal immunity instructed to commensals, pathobionts and pathogens. He received a Career Development Award from the Austrian Academy of Sciences in 2008, and a Research Achievement Award for the research publication with the highest impact (McGill University, 2012) and a Research Scholar Junior 1 Award by the Fonds de recherche du Québec – Santé (FRQS).

Silvia Vidal
Co-DirectorSilvia Vidal, PhD, is a Professor, Department of Human Genetics, McGill University and an Associate Member of the Department of Microbiology and Immunology, and is Director of the CTG. She holds a Tier 1 Canada Research Chair in Host Responses to Virus Infections and is the recipient of the Premier’s Research Excellence Award. She uses mouse genetic platforms to discover and functionally characterize the molecular interface between pathogenic viruses, inflammation and immunity. Her laboratory made inroads in characterizing mechanisms of self/non-self discrimination by NK cells during viral infection and pathways that control inflammatory responses during coxsackieviral myocarditis and influenza pneumonia. She has also developed an internationally recognized program in ENU mutagenesis and infectious diseases. Vidal’s program has spearheaded a number of projects and collaborations with academia and industry at McGill and abroad on gene discovery in human infectious and inflammatory disorders.
MRCCT members by Research Streams
 Host Microbe Interaction in Health and Disease
Host Microbe Interaction in Health and Disease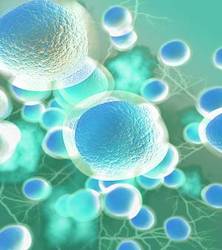 Cellular Dynamics of Immune Dys(function)
Cellular Dynamics of Immune Dys(function) Computational and Genomic Medicine
Computational and Genomic Medicine
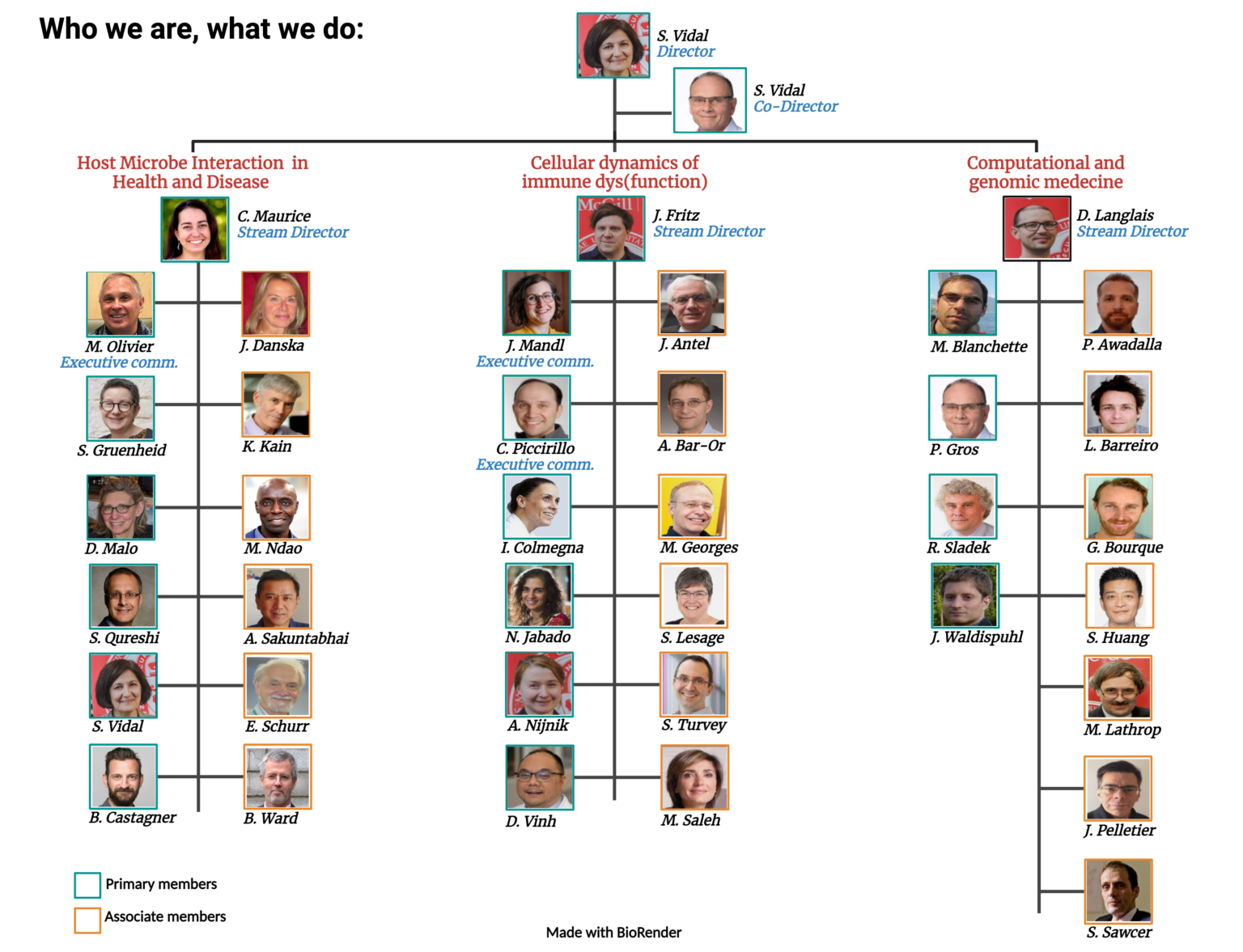
Our Members

Corinne Maurice
Stream DirectorDr Corinne Maurice is an Assistant Professor in the Department of Microbiology and Immunology at McGill University, a Tier 2 Canada Research Chair in gut microbial physiology, and an Azrieli Global Scholar. Her work aims to identify how to manipulate the human gut microbiome for health benefits, by characterizing the active bacterial subset of the human gut microbiome and determining how bacteriophages regulate it. Her expertise in single-cell tools and metagenomics have allowed her to pioneer the FACSeq method, whereby bacterial cells with distinct activity or damage can be sorted and sequenced to better characterize functional changes in this dynamic community. By applying microbial ecology concepts and tools to the study of bacteria and phage interactions, her group is currently exploring the role of bacteriophages in health, child stunting, and adult IBD.

Samantha Gruenheid
Executive comm.Dr. Samantha Gruenheid, PhD, is an Associate Professor, Department of Microbiology and Immunology, McGill University and a FRQS Senior Chercheur-Bousier. Her expertise is in the field of host-pathogen interactions, with a particular focus on intestinal infections. Dr. Gruenheid investigates bacterial virulence mechanisms and host responses to infection. Applying a genetic approach to infection with the mouse intestinal pathogen C. rodentium, her group recently discovered a new link between intestinal infection/inflammation and the regulation of tissue homeostasis by Wnt signaling. Her lab has also made important contributions towards the elucidation of the cell biology underlying virulence mechanisms of pathogenic E. coli.

Martin Olivier
Executive comm.Dr. Martin Olivier, PhD. The Laboratory of Martin Olivier (Departments of Medicine, Microbiology/Immunology) focuses on understanding how pathogens for major global infectious diseases (Malaria, Leishmaniasis, Trypanosomiasis, Phleboviruses) can influence the host innate inflammatory response by manipulating the biochemical cascades involved in the regulation of phagocyte functions. Using in vitro and in vivo infectious models we are investigating how extracellular vesicles (e.g. exosome) released by protozoan parasites in culture or within their blood-sucking vectors contribute to facilitate the infection and its propagation within the mammalian host. In addition, impact of virus/Leishmania co-infection on cutaneous leishmaniasis development and the immunological events underlying this co-infection is studied using various KO mice for major innate immune response sensors (TLR2/RIG-I/MAVS). Using our exosome biology expertise, we are interested to isolate and characterize those vesicles from various biological fluids of human affected by malaria, visceral leishmaniasis and tuberculosis. Therefore, permitting to better understand the pathologies of these infections and to develop exosome-based biomarker diagnostics and vaccines for those infectious agents. Finally, my lab is actively developing and testing new molecules capable to induce or inhibit host protein tyrosine phosphatases to block inflammatory-based pathologies or to modulate protective immune response against infectious agents.

Danielle Malo, DVM
Dr. Danielle Malo, MV, PhD, is a veterinarian and a Professor in the Departments of Medicine and Human Genetics, and a founding member of the McGill University Research Centre on Complex Traits (MRCCT). She is the co-Director of The Centre for Phenogenomics (TCP) Infection and Inflammation Core at McGill University (https://www.mcgill.ca/mrcct/tcp-infection-inflammation-core ) and the co-Director of the McGill containment level 3 platform. She uses mouse models of infection and genetic platforms to discover and characterize genes and pathways that control the host response to intracellular pathogens. She discovered and characterized the function of several innate immune genes in response to Salmonella and Mycobacterium tuberculosis infections, among those Toll-like receptor 4 (TLR4), Ubiquitin specific protease 18 (USP18) and CYFIP Related Rac1 Interactor B (CYRIB). More recently she was involved in the activation of SARS-CoV2 research at McGill.

Salman Qureshi
Dr. Salman T. Qureshi, MD, is an Associate Professor, Department of Medicine and Research Director at the Meakins-Christie Laboratories as well as an attending physician in the Department of Critical Care and medical director of Adult Respiratory Therapy at the McGill University Health Centre. He is a recipient of a Tier 2 Canada Research Chair in Host Response to Respiratory Infections. He uses forward genetic strategies to identify and functionally characterize the molecular determinants of susceptibility to pathogenic fungi, bacteria, and viruses that infect the lung including the identification of several loci that mediate host defense against Cryptococcus neoformans. He contributed to the initial development of an ENU mutagenesis program platform at McGill to probe host susceptibility to infectious diseases. The overall aim of his research program is to lay a foundation for the development of targeted therapies of human infectious and inflammatory disorders.

Silvia Vidal
Co-DirectorSilvia Vidal, PhD, is a Professor, Department of Human Genetics, McGill University and an Associate Member of the Department of Microbiology and Immunology, and is Director of the CTG. She holds a Tier 1 Canada Research Chair in Host Responses to Virus Infections and is the recipient of the Premier’s Research Excellence Award. She uses mouse genetic platforms to discover and functionally characterize the molecular interface between pathogenic viruses, inflammation and immunity. Her laboratory made inroads in characterizing mechanisms of self/non-self discrimination by NK cells during viral infection and pathways that control inflammatory responses during coxsackieviral myocarditis and influenza pneumonia. She has also developed an internationally recognized program in ENU mutagenesis and infectious diseases. Vidal’s program has spearheaded a number of projects and collaborations with academia and industry at McGill and abroad on gene discovery in human infectious and inflammatory disorders.

Bastien Castagner
Dr. Bastien Castagner obtained a Ph.D. in Chemistry at Columbia University in New York in 2004. His postdoctoral years were spent at ETH Zürich from 2005 – 2008. From 2009 – 2014 he was a Group Leader in the Institute of Pharmaceutical Sciences at ETH Zürich. He joined the Department of Pharmacology & Therapeutics at McGill in 2014 and was promoted to the rank of Associate Professor in 2020. His research focuses on the design of small-molecules and natural product analogues as novel drug candidates. He is especially interested in the chemistry and biology of inositol phosphates and carbohydrates. His group has been involved in novel strategies to inactivate the toxins responsible for the pathogenesis of Clostridioides difficile. He is also interested in the human gut microbiota and how molecules can impact its composition and metabolism, for the benefit of the host (us!).

Jayne Danska
Associate MemberJayne Danska, PhD, is the Anne and Max Tanenbaum Chair in Molecular Medicine, a Senior Scientist at the Hospital for Sick Children, and a Professor, Departments of Immunology and Medical Biophysics, Faculty of Medicine, University of Toronto and . She has made contributions to understanding immunological, genetic and environmental causes of Type 1 diabetes (T1D), molecular mechanisms of acute lymphoid leukemia (ALL), and innate immune surveillance of leukemia. She leads large-scale projects applying genetic, genomic and immunological analysis to identify T1D-risk genes and the role of the intestinal microbiome as a modifier of risk for immune-mediated disease. Her group demonstrated that manipulation of the intestinal microbiome influences sex hormone levels metabolism and modifies a high burden of inherited risk in a mouse model of spontaneous T1D. Her work on identification of innate immune checkpoints and industry partnership led to development of a novel biologic inhibitor that performed well in early trials for hematologic malignancies. In 2021, the checkpoint inhibitor was acquired by Pfizer to advance into phase 3 trials in multiple malignancies.

Kevin Kain
Associate MemberKevin Kain, MD, FRCPC, is a Professor of Medicine, University of Toronto; the Director, SAR Laboratories, Sandra Rotman Centre for Global Health; Science Director, Tropical Disease Unit at UHN-Toronto General Hospital; and holds a Canada Research Chair (Tier I). Dr. Kain’s research efforts are focused on new diagnostics and interventions for major global infectious disease threats including malaria, sepsis and HIV, particularly as they pertain to maternal-child health. Dr. Kain is the recipient of the C. Woolf Award for the Excellence in Teaching from the University of Toronto, a Career Scientist Award from the Ontario Ministry of Health, the Bailey Ashford Medal from the American Society for Tropical Medicine, awarded for “Distinguished work in tropical medicine”, and the SL Medal (from Mahidol University, Thailand) for “Outstanding achievements in malaria research”. Dr Kain was profiled by TIME magazine as one of “Canada’s Best in Medicine”. He has received the: Pfizer Visiting Professorship, University of Washington; Fred Barrett Lectureship, University of Tennessee; Distinguished Global Health Service Award, University of California; The Henry Wong Lectureship in Medicine, McMaster University; Forbes Lectureship, University of Melbourne. He has served as a consultant to many organizations including the Gates Foundation, WHO, Red Cross, CBS, PATH and the CDC.
Peer-reviewed Research Support: totals over $100,000,000. Peer reviewed Publications: over 375. Peer-reviewed Research Support: totals over $75,000,000. Peer reviewed Publications: over 300.

Momar Ndao
Associate MemberDr. Momar Ndao research focuses on bringing cutting edge science to diagnose infectious diseases, studying host-parasite interactions, screening drugs to be used as therapies for infectious diseases, developing vaccines to prevent parasitic diseases, and applying proteomic technology to discover biomarkers for infectious diseases. Increased awareness of parasitic infections has led to newly recognized parasites, emerging pathogens, and bioterrorism considerations. Therefore, the fields of diagnostic medical parasitology treatment and vaccines are undergoing dramatic change. His laboratory has interests in 1) diagnosis of parasitic diseases 2) the study of host-parasite interactions; 3) screening drugs to be used as therapies for protozoan parasitic disease; 4) developing vaccines to prevent parasitic diseases and 5) applying proteomic technology to discover biomarkers for infectious diseases.

Anavaj Sakuntabhai
Associate MemberAnavaj Sakuntabhai, MD, and Professor at the Institut Pasteur, Paris where he was recruited in 2000 to develop a program on the genetics of infectious diseases. He discovered a variant on a promoter of DC-SIGN associated with gene expression and outcome of Dengue virus infection. He published an important finding of positive selection of G6PD (glucose 6 phosphate dehydrogenase) and its effect on Plasmodium vivax density in Science. His recent research has shown that both gene-gene and gene-environmental interactions play a significant role in susceptibility to malaria and Dengue fever. He successfully coordinated two important projects on genetic susceptibility to malaria and dengue involving teams from France, Thailand, and Senegal. He coordinated a global network for Dengue fever research for the Institut Pasteur International Network. He proved that asymptomatic dengue infected individuals could transmit the virus to mosquito vector, which have huge impact on dengue surveillance and control. In addition, He found that T cells are highly activated in asymptomatic as compared with symptomatic infections. The last two findings lead to a new concept of monovalent dengue and Zika vaccine development. He has significant experience in coordination of international programs, having been coordinator of European FP7 project on Dengue Framework for Resisting Epidemics in Europe (DENFREE). He was involved in investigating two recent global outbreaks of infectious diseases, Ebola and Zika. Currently, he is a leader of the Pasteur International Center for Research on Emerging Infectious Diseases (PICREID) – a multi-million dollars program supported by the NIH. The project is implemented West, Central Africa and South-East Asia, linking large observational multicenter cohort studies with basic scientific research and leading to increased preparedness for new epidemic threats in the region. Recently, he was appointed as the director of Institut Pasteur Japan office to establish transdisciplinary network on Emerging Infectious Diseases between Japan, France and countries in Indo-Pacific region.

Erwin Schurr
Associate MemberErwin Schurr obtained his Ph.D. in 1986 from the Institute of Biophysics and Radiation Biology at the Albert-Ludwigs University in Freiburg/Br, Germany. He then did his postdoctoral studies in molecular genetics with E. Skamene and P. Gros at McGill University. In 1991, he joined the McGill Centre for the Study of Host Resistance and the Faculty of Medicine at McGill as Assistant Professor. He is a Distinguished James McGill Professor of Human Genetics and Medicine at McGill University. At the Research Institute of the McGill University Health Centre he is the leader of the program on Infectious Diseases and Immunity in Global Health. His main research interest is the identification of host genetic factors predisposing to tuberculosis and leprosy; research that is supported by both national and international funding agencies. He has been involved in numerous field studies in main endemic countries, and he has published extensively on the human genetics and genetic epidemiology of both leprosy and tuberculosis.

Brian Ward
Associate MemberBrian Ward, PhD, is a professor of Medicine & Microbiology at McGill University, Deputy Director of the Research Institute of the McGill University Health Centre (Fundamental Science), Co-Director of the McGill Vaccine Study Centre and Associate Director of the JD MacLean Center for Tropical Diseases. His laboratory is currently active in three areas: 1) the immunologic evaluation of vaccines and vaccine safety, 2) the evaluation of micronutrient-microbial interactions, and 3) the development of novel therapeutic strategies for microbial pathogens. Although a good deal of the work performed in the laboratory takes place in Montreal, many of the projects have significant or even predominant components overseas. There is a long-standing commitment in the laboratory to collaborative work with developing world researchers in Peru and Zimbabwe. Dr. Ward serves on a wide range of government and industry advisory committees related to vaccines, vaccine safety, international health and parasitology.

Jorg Fritz
DirectorDr. Jörg Hermann Fritz, PhD, is a CIHR New Investigator, and an Associate Professor (Departments of Microbiology and Immunology and Physiology). He is an immunologist who trained with Dr. D. Philpott (Institut Pasteur, Paris), and Dr. J. Gommerman (U. Toronto), and who joined the MRCCT in 2010. Research in his laboratory focuses on understanding how tissue homeostasis at mucosal surfaces is regulated in health and disease. A particular focus is given to studies of mucosal pathogens of the respiratory and gastrointestinal system where he is trying to understand how the expression pattern and activity of innate resistance effectors adapts to changes in the tissue milieu due to the availability of nutritional metabolites, the composition of the mutualistic microflora, or infection with pathogens. In this context he is studying the priming and function of innate lymphoid cells and B lineage cells for their role in mucosal immunity instructed to commensals, pathobionts and pathogens. He received a Career Development Award from the Austrian Academy of Sciences in 2008, and a Research Achievement Award for the research publication with the highest impact (McGill University, 2012) and a Research Scholar Junior 1 Award by the Fonds de recherche du Québec – Santé (FRQS).

Judith Mandl
Executive comm.Dr. Judith Mandl, Ph.D., is an Associate Professor in the Department of Physiology, an associate member in the department of Microbiology and Immunology, and a primary member of MRCCT. She currently holds a Tier 2 Canada Research Chair in Immune Cell Dynamics and was recently awarded the New Investigator Award by the Canadian Society for Immunology. Since starting her lab at McGill in 2015, she has built on her expertise in cellular immunology, studying the complex migration choreography of immune cells that is critical to effective immune responses, with a particular focus on T cells. Her research focuses on the specific challenges T cells face as they move in tissues, to what extent nuclear deformability and cytoskeletal processes define how T cells make decisions as they navigate, the consequences of perturbing immune cell migration for T cell function and differentiation, and the specific interactions made by T cells during their migration which leads to heterogeneity in their behaviour. Her lab applies state-of-the-art microscopy and systems biology tools, using mouse models to link aspects of individual immune cell migratory behaviour to both within-cell cytoskeletal processes and whole organismal-level readouts such as cell differentiation, homeostasis and responses to infection.

Ciro Piccirillo
Executive comm.Dr. Ciriaco Piccirillo, PhD, is an Associate Professor, Department of Microbiology and Immunology, McGill University Health Centre. He is an immunologist who trained at the reputed Laboratory of Immunology, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). He is currently Principal Investigator of the Laboratory of Immunoregulation unit at the Research Institute of the McGill University Health Center (RI-MUHC) and co-Leader of the Infection and Immunity Axis at the RIMUHC. He is also Director of the Immunophenotyping platform at the RI-MUHC and the Director of McGill’s FOCIS Center of Excellence in Translational Immunology and Therapeutics whose mission is to support basic and clinical research in Immunology.

Ines Colmegna
Dr. Ines Colmegna, MD, is an Associate Professor in the Division of Rheumatology and a Clinician Scientist at the Research Institute of the McGill University Health Centre. She is Affiliated to the Department of Microbiology and Immunology and Experimental Medicine. She holds a FRSQ Chercheur-Boursier Senior. Her laboratory studies the impact of aging on stem cell function to understand how immune aging favors autoimmunity. Her clinical focus is rheumatoid arthritis and she is involved in National and International initiatives that relate to this disease.

Nada Jabado
Dr. Nada Jabado, MD, is a Professor of Pediatrics at McGill University and pediatric neuro-oncologist at the Montreal Children’s Hospital. She began her career as an independent investigator at McGill in 2003, pioneering a research program in pediatric brain tumours which is now unparalleled. Dr. Jabado’s group was among the first to identify a histone mutation in human disease which has revolutionized this field. The epigenome was a previously unsuspected hallmark of oncogenesis and this discovery linked development and what we now know are epigenetic-driven cancers. Dr. Jabado has over 220 peer-reviewed publications to her credit, with an impressive number of senior-author, high-impact publications in such prominent journals as Nature, Science and Cell. She is an international leader in the field of neuro-oncology/cancer, honored by invitations as a keynote speaker at top ranked symposia and universities. Dr. Jabado has been inducted as a Fellow to the Royal Society of Canada, is an appointed member of the CIHR Governing Council, and holds a Tier 1 Canada Research Chair in Pediatric Oncology.

Ana Nijnik
Dr. Ana Nijnik is a Canada Research Chair Tier II in Hematopoiesis and an Associate Professor at the Department of Physiology. Dr. Nijnik completed her undergraduate studies and Ph.D. at Oxford University (UK), followed by postdoctoral training at the University of British Columbia (Canada) and the Wellcome Trust Sanger Institute (Cambridge, UK), specializing in immunology, stem cell biology, and genetics. Dr. Nijnik and her research team investigate the process of blood and immune cell differentiation from hematopoietic stem cells (HSCs) in health and disease, and their work has broad implications for the understanding of the disorders affecting the bone marrow, blood, and immune system, including bone marrow failures, primary immunodeficiencies, inflammatory disorders, and hematologic malignancies. Specifically, current research projects focus on the transcriptional and epigenetic regulation of hematopoiesis and immunity by chromatin associates deubiquitinases (DUBs), and on the regulation of hematopoietic responses to genotoxic and inflammatory stress. Dr. Nijnik is also a member of the McGill Regenerative Medicine Network, and teaches in several upper-level courses for the Department of Physiology.

Don Vinh
Dr. Donald Vinh, MD, is an Associate Professor, Division of Infectious Diseases and in the Division of Allergy & Clinical Immunology (Department of Medicine), as well as in the Division of Medical Microbiology (Department of Pathology and Laboratory Medicine), McGill University Health Centre. He is also an Affiliate member of the Department of Human Genetics. He currently holds a chercheur-boursier clinicien Senior award from the Fonds de recherche du Québec-Santé (FRQS) for his translational research program focusing on defining inborn errors of immunity in humans: By combining genetic approaches with functional immunology on robustly-defined patient cohorts, his bedside-to-bench research aims to understand the molecular mechanisms that confer susceptibility to infections, particularly fungal and viral diseases. One of the main research focus of his laboratory is genetic susceptibility to fungal infections: His lab is at the forefront of defining the genetic epidemiology of CARD9 deficiency in Québec and dissection of its molecular underpinnings that have therapeutic applications, a program for which he was awarded the AMMI Young Investigator Award (2016). The other main focus of his program is on the discovery and characterization of combined immunodeficiency syndromes. His lab has discovered ICOSL deficiency, due to biallelic mutations in ICOSLG, and is mapping out the functions of this molecule in human immunity.

Jack Antel
Associate MemberJack Antel, MD, is a Professor, Department of Medicine, McGill University Health Centre. He examines how the immune system interacts with cells in the central nervous system. His work deals with immune-mediated neurological diseases, especially multiple sclerosis. His studies with human tissues are often designed in parallel with the mice models used by his colleague, Dr. Trevor Owens, who studies experimental allergic encephalomyelitis (EAE). Another focus of his research is to understand how cells of the immune system injure oligodendrocytes and their myelin membranes. He is also examining how glial cells (astrocytes and microglia) serve as antigen-presenting cells that regulate T-cell reactivity.

Amit Bar-Or
Associate MemberAmit Bar-Or, MD is a practicing neuroimmunologist, Montreal Neurological Institute and Hospital. He also serves as Director, Experimental Therapeutics Program and Scientific Director, Clinical Research Unit at the MNI. His lab studies basic principles of immune-regulation, immune-neural interaction and neural-glial interaction, and roles in physiologic processes, inflammatory injury and repair in the human central nervous system. His clinical focus is multiple sclerosis (MS) and he is currently the President of the Canadian Consortium of MS Clinics. He coordinates a number of multi-center national and international translational research initiatives. An overarching theme is translation of basic lab discoveries towards development and understanding of novel experimental therapies and biomarkers for patients with autoimmune and neurological diseases. He serves on several journal editorial boards and on the scientific/advisory boards of the Guthy-Jackson Greater-Good Foundation; the Accelerated Cure Project; the ACTRIMS, ISNI and FOCIS organizations.

Michel Georges
Associate MemberMichel Georges, PhD, is a Professor in Genetics and Genomics, Faculty of Veterinary Medicine, University of Liège, Belgium. He heads the Unit of Genetics of the GIGA (Groupe Interdisciplinaire de Génoprotéomique Appliquée) Research Institute in the same university. He played an instrumental role in establishing the GIGA Research Institute. He devoted his scientific carrier to the development and use of genomic tools for the identification of genes and mutations underlying complex traits of agronomic and medical importance. He participated in the very first genome scans for QTL in the rat, and then conducted many such scans in livestock. His lab has discovered polar overdominance, identified the “double-muscling” gene and several regulatory QTN including some that perturb miRNA-mediated gene regulation, and discovered a novel CNV generating mechanism underlying the inheritance of colour-sidedness. He made important contributions to the “genomic selection revolution” in livestock. More recently, Georges’ team has been involved in the genetics of inflammatory bowel disease and has contributed to the identification of novel risk loci, genes and variants.

Sylvie Lesage
Associate MemberSylvie Lesage, PhD, is a Full Professor at l’Université de Montréal in the Département de microbiologie, infectiologie et immunologie and is a Research Scholar Emeritus from the Fonds de recherche Québec – Santé. In June 2021, she was appointed Scientific Director of the Centre de recherche de l’Hôpital Maisonneuve-Rosemont (CRHMR). Dr. Lesage obtained her Ph.D. at McGill University under the supervision of Dr. Patrice Hugo, where she gained expertise in immune tolerance. She then continued her postdoctoral training with Dr. Christopher C. Goodnow in Australia, where she applied the concepts of immune tolerance in mouse models of type 1 diabetes. During her second postdoctoral training in Montreal, Dr. Lesage learned to transpose the knowledge of the mouse to humans. She then established an independent research laboratory at the CRHMR, where her team focuses on cellular immunogenetics. Specifically, they strive to understand the homeostatic dynamics of different immune cell populations linked to the predisposition to complex genetic diseases. These studies will serve as a springboard for translational research aimed at restoring immune balance to prevent the onset or to slow the progression of autoimmune diseases and cancers. Her success is noted by more than 85 career publications, and her research program is funded by various organizations, including the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, the Cancer Research Society, and the Grand défi Pierre Lavoie Foundation.

Stuart Turvey
Associate MemberStuart Turvey, MBBS, DPhil, FRCPCis a Professor of Pediatrics at The University of British Columbia where he holds both the Canada Research Chair in Pediatric Precision Health and the Aubrey J. Tingle Professorship in Pediatric Immunology. He is a clinician-scientist and Pediatric Immunologist based at BC Children’s Hospital. He completed both his Pediatric Residency and Allergy/Immunology Fellowship at Children’s Hospital, Harvard Medical School, Boston. He holds a medical degree (MB BS) from the University of Sydney, Australia and a doctorate (DPhil) in Immunology from Oxford University where he was a Rhodes Scholar. Dr. Turvey is a Fellow of the Royal College of Physicians and Surgeons of Canada and a Diplomate of the American Board of Pediatrics. His research program is focused on determining the early-life origins of asthma and allergic disease, as well as harnessing the power of genomics to diagnose, treat and prevent pediatric immune disorders. His ability to transition from the clinic to the lab allows Dr. Turvey to take a precision health approach that is sensitive to the developmental course of the child. His work determines and responds to the underlying cellular, molecular and genetic abnormalities responsible for disease. Dr. Turvey and his team generate new knowledge to define the mechanisms of disease pathogenesis and, ultimately, to identify new strategies to treat and even prevent these burdensome and often life-threatening conditions.

David Langlais
Stream DirectorDr. David Langlais is an Assistant Professor in the Department of Human Genetics at McGill University and Principal Investigator at the MRCCT and McGill Genome Centre. Dr Langlais completed his Ph.D. with honours in Molecular Biology under the supervision of Dr. Jacques Drouin at the Institut de recherches cliniques de Montréal. His work revealed the complex transcriptional regulation and tissue maintenance mechanisms at the immuno-neuroendocrine interface. Dr Langlais then pursued postdoctoral research in Dr Philippe Gros’ laboratory at McGill University where he studied the role of critical innate immunity transcription factors and participated in the characterization of new genes involved in immune functions. Dr Langlais is a FRQS Chercheur-Boursier Jr1 and has received multiple awards, including the Prof Kenneth Morgan Teaching Award and the Milstein Young Investigator Award form the International Cytokine and Interferon Society. His current research is founded on functional genomics, bioinformatics, genome editing and molecular biology methods to explain the transcriptional mechanisms involved in normal and pathological inflammatory responses, aiming to identify and validate novel therapeutic targets for inflammatory diseases.

Mathieu Blanchette
Dr. Mathieu Blanchette, PhD, Associate Professor, School of Computer Science, McGill Centre for Bioinformatics, McGill University. His lab focuses on the development of algorithmic and machine learning approaches to biological sequence analysis. He is interested in the analysis of transcriptional regulation using approaches based on comparative genomics. He also develops computational approaches to the analysis of chromosome conformation capture data.

Philippe Gros
Philippe Gros, PhD, Deputy Vice-Principal, Research and innovation, McGill University. Dr. Gros is a Distinguished James McGill Professor of the Department of Biochemistry, and founding member of the McGill Center for the Study of Complex Traits. He is also a member of the McGill International Center for Tuberculosis, and the Goodman Cancer Research Center, and is an associate member of the Departments of Human Genetics, and Microbiology and Immunology. His main area of investigation concerns the genetic analysis of susceptibility to infectious diseases such as malaria and tuberculosis, inflammatory conditions such as inflammatory bowel diseases and multiple sclerosis, and neurodegenerative diseases such as Parkinson’s disease. He has received several prestigious awards since 2008 including, the Wilder Penfield Prize (Gouvernement du Québec; 2008), the Killam Prize in Health Sciences (Canada Council of the Arts; 2008), the Champion of Genetics Awards (GeneCure Foundation; 2011), the Queen Elizabeth II Diamond Jubilee Medal (Association of Universities and Colleges of Canada, 2013), the McLaughlin Medal (Royal Society of Canada, 2014), the Canadian Institutes of Health Research, Chair, College of Reviewers (one of fifteen/2016), Officer, Order of Canada (2016), and was named in the Quebec Sciences magazine, one of the Top 10 discoveries of 2018, and was elected Chevalier de l’Ordre National du Québec in 2019. He acts as an advisor for several organizations, including the Canadian Institutes of Health Research, the Canadian Institute for Advanced Research and is a member of the Canadian Covid-19 Immunity Task Force. His experience in the biotechnology sector, includes the co-founding of PhageTech, Emerillon Therapeutics (Xenon), and Corbin Therapeutics.

Rob Sladek
Dr. Rob Sladek is an Associate Professor of Human Genetics and Medicine (Endocrinology) at the McGill University and Génome Québec Innovation Centre. He completed undergraduate degrees in Engineering Science and Medicine and a clinical fellowship in Endocrinology, all at the University of Toronto. His postdoctoral training explored the role of the Estrogen-related receptors in the regulation of fat metabolism (Err-alpha) and trophoblast formation (Err-beta); as well as and the application of high-throughput genomics technologies to study complex traits. He leads the Diabetes Gene Discovery Group – a project to identify risk loci for Type 2 diabetes that was sponsored by Genome Canada and Génome Québec. Rob’s current research centers on developing and applying new technologies to study gene transcription networks and protein function in living cells in order to understand how genetic risk loci influencing T2D risk or the host response to tumors exert their effects.

Jérôme Waldispühl
Dr. Jérôme Waldispühl is an Associate Professor at the School of Computer Science at McGill. He conducts research in computational molecular biology with a strong emphasis on RNA sequence/structure analysis and protein folding prediction. Recently, he also gained interests in applications of crowdsourcing and human-computing techniques in bioinformatics. He is also recognized for his pioneering work in crowdsourcing genomics. He leads the Computer Science & Biology Group at the School of Computer Science of McGill University. He holds a PhD in Computer Science from École Polytechnnique (France) and previously was a instructor in applied mathematics at the Massachusetts Institute of Technology. He is a recipient of the Tomlinson Scientist Award (2012) and the Fessenden Professorship in Science Innovation in (2013).

Philip Awadalla
Associate MemberPhilip Awadalla, PhD, is a Senior Investigator at the Ontario Institute for Cancer Research, Professor of Population and Medical Genomics at the University of Toronto, and is a Principal Investigator of the Canadian Partnership for Tomorrow Project and biobank. Since his training at the University of Edinburgh, Dr. Awadalla has focused on developing experimental and computational tools to study fundamental processes in genomics in humans and pathogens. His research investigates broad range of chronic and rare diseases, including infectious diseases in the developing world. Dr. Awadalla is also the Principle Investigator and Director of the CARTaGENE Biobank of Quebec. CARTaGENE is a prospective public health survey of Quebec and in its first phase captured biological, clinical, genealogical and genomic data from over 20,000 participants. He is also co-director of the Centre for Child Health Genomics at University of Montreal and he currently holds the Genome Quebec recruitment award for Population and Medical Genomics. He was awarded the 2012 Joe Doupe Young Clinical Investigator of the year.

Luis Barreiro
Associate MemberLuis Barreiro, PhD. In July 2018, the Barreiro lab officially moved to the University of Chicago. He was an Assistant Professor, Département de biochimie et médecine moléculaire, Université de Montréal from 2011 to 2018. From 2003 to 2008 he worked at the Pasteur Institute of Paris where he did his PhD in Human Population Genetics under the supervision of Lluis Quintana Murci. In 2008 he moved to the department of Human Genetics at the University of Chicago to do a post-Doc in functional genomics. Luis Barreiro started his own laboratory at the University of Montreal and the CHU Ste-Justine in March 2011. His research focuses on a better understanding how natural selection has contributed to the evolution of our species and the extent to which past selection events impact present-day susceptibility to disease. Specifically, Barreiro’s lab studies the evolution of immune responses both at the inter-species level as well as among different individuals and human populations.

Guillaume Bourque
Associate MemberGuillaume Bourque, PhD is a Professor, Department of Human Genetics, McGill University, a Canada Research Chair in Computational Genomics and the Director of Bioinformatics at the McGill Genome Center (MGC). He is the lead of the CIHR-funded Epigenomic Data Coordination Center at McGill. His research interests are in comparative and functional genomics with a special emphasis on applications of next-generation sequencing technologies. Dr. Bourque has a close partnership with Calcul Québec to implement computational genomics within a secure high-performance computing environment.

Sid Huang
Associate MemberSidong Huang, PhD, is an Associate Professor, Department of Biochemistry, McGill University. He is also a Member of the Rosalind and Morris Goodman Cancer Institute and holds a Canada Research Chair in Functional Genomics. Using functional genomic tools to study cancer-relevant pathways and to guide targeted cancer therapy, his research has uncovered novel resistance mechanisms to cancer therapeutics, identified new treatment strategies, and led to the establishment of clinical trials. One of his studies has led to a recently approved drug combination co-targeting BRAF and EGFR to treat metastatic BRAF mutant colorectal cancer patients. He is also the scientific director of the McGill Platform for Cellular Perturbation, which provides McGill researchers with low-cost and rapid access to the latest functional genetic tools for biological research.

Mark Lathrop
Associate MemberMark Lathrop, PhD, is the Scientific Director at McGill University and Genome Quebec Innovation Centre. Renowned Canadian genomics pioneer, Mark Lathrop has been most recently the scientific director of the Centre National de Genotypage (CNG) and of the Fondation Jean Dausset Centre d’Étude du Polymorphism Humain (CEPH) in Paris, two of the major Centres for large scale biological research established by the French government. The principal goal of these Centres is to apply genomics and other large-scale methodologies to understanding human disease. He has also made major contributions to genetic approaches for the study of models of human disease in other mammalian species. His present scientific studies focus on using genetic and other high-throughput genomic approaches to identify DNA variants that predispose people to common diseases, particularly, lung cancer, asthma and cardiovascular disease, and to understand the effects of these in a biological and public health context. He is responsible for the scientific program of the French National Programme in Cancer Genomics.

Jerry Pelletier
Associate MemberJerry Pelletier, PhD is a James McGill Professor, Department of Biochemistry and Oncology and member of the Rosalind and Morris Goodman Cancer Research Centre, McGill University. The overarching focus of his research program is to understand how translation, a process fundamental to all cells, becomes deregulated in disease – ranging from orphan diseases to cancer to neurological disorders. Dysregulation of mRNA translation is a frequent feature of neoplasia and Pelletier’s studies have identified nodal points that are druggable vulnerabilities as well as unique small molecule inhibitors that interdict this process. He has also developed mouse cancer models that mimic small molecule-mediated targeted inhibition at the organismal level and have used these to validate the concept of targeting translation initiation in vivo. Furthermore, he has developed powerful methods for applying genome engineering technology (CRISPR-Cas9) to link the biological activity of small molecules to target engagement. He has experience in leading large collaborative research programs (CQDM, CIHR Team Grants, CFI, Terry Fox Research Institute) and has forged strong collaborative links with academic and industry on small molecule targeting to deregulated translational control. He has an internationally recognized research program that integrates powerful mouse cancer models, chemical biology, and genome engineering to explore the role of translation in tumor maintenance and cell death mechanisms and characterize their impact on treatment response.

Stephen Sawcer
Associate MemberStephen Sawcer, PhD FRCP, Professor of Neurological Genetics, University of Cambridge, and an Honorary Consultant Neurologist at Addenbrooke’s Hospital. Dr. Sawcer uses genome wide association screening to identify genetic variants associated with disease susceptibility and clinical phenotype. To date these efforts have identified over 200 variants associated with susceptibility to multiple sclerosis that have confirmed the immunological nature of the disease and provided targets for functional analysis. Within the UK, he runs a National Institute of Health Research portfolio project recruiting patients with multiple sclerosis into genetic studies that has involved over 50 Centres. Internationally Dr. Sawcer has led or co-led several of the largest genetic studies ever performed in multiple sclerosis. Dr. Sawcer spent his sabbatical in Canada working with Dr. Wee Yong in Calgary and has extensive collaborations with the McGill and Genome Québec Innovation Center in Montreal.